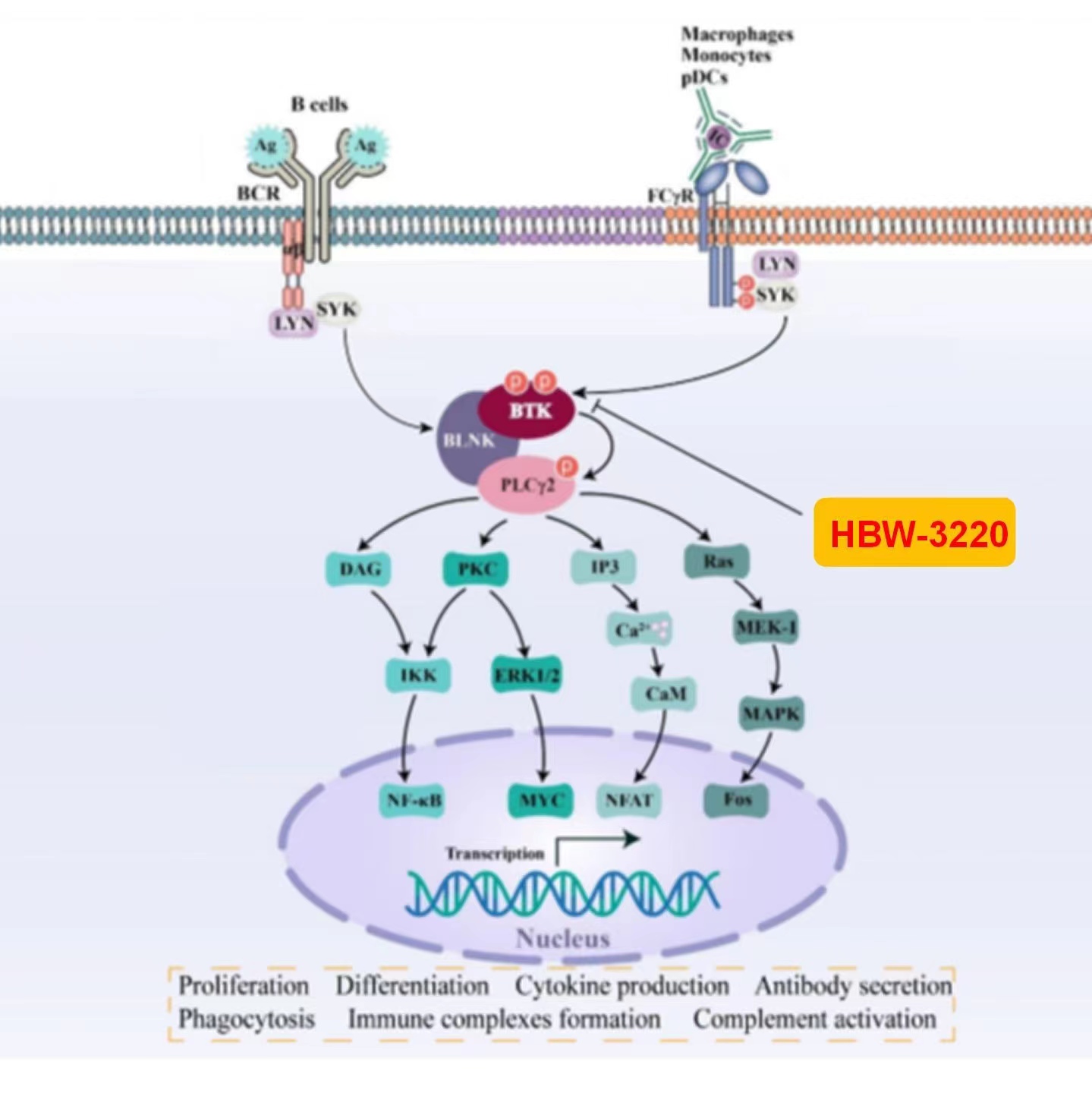
 HBW-3220
HBW-3220
BTK (Bruton's tyrosine kinase), a central regulator of the B cell receptor signaling pathway, has been targeted for treating a variety of B cell malignancies and autoimmune diseases, such as systemic lupus erythematosus and multiple sclerosis. Currently, there are 6 BTK inhibitors (BTKi) approved in the world. BTKis’ global market has exceeded US $10 billion and will reach US $26.1 billion by 2030 as estimated. The use of the 1st and 2nd generation covalent BTK inhibitors (BTKis) has often resulted in the development of resistance, commonly associated with the mutation at the cysteine 481 where the inhibitor binds covalently; therefore, a non-covalent BTKi will offer greater therapeutic benefit. Hyperway has developed two novel 3rd generation reversible BTKis, HBW-3220 and HBW-3210, respectively, that can bind potently to BTK wild-type and several BTK mutations, including the most common mutation C481S.
HBW-3220 is currently in the clinical phase I study for treating B-cell malignancies. In our preclinical studies, HBW-3220 has been compared head-to-head with many approved/clinical drugs , and demonstrated its superiority in efficacy, safety, and pharmacokinetic properties. On March 31, 2023, Hyperway added another IND application for a new indication of nephropathy.
HBW-004285 
Nav1.8 is a tetrodotoxin-insensitive sodium channel mainly expressed in nociceptive neurons. It plays a key role in pain signal transduction in the peripheral nervous system and presents a promising target for pain therapy. Currently, the major challenge for treating pain is the development of addiction, which is unavoidable by opioid-related therapeutics. Inhibiting Nav1.8 provide a new non-addictive mechanism and has no effect on motor function. Worldwide, there are only limited Nav1.8 inhibitors have been developed.
Hyperway’s HBW-004285 is one of the forerunners. In several preclinical head-to-head studies using animal models, HBW-004285 is superior to competitors in safety, efficacy, PK, and toxicity data. Importantly, HBW-004285 does not require prodrug modification, a great advantage over the other competitors! The preclinical results were selected to be published as an abstract by American Neurological Association (2021).
On April 11, 2023, HBW-004285 obtained an implied license for clinical trials for treating acute and chronic pain. The first patient was enrolled on April 26, 2023.
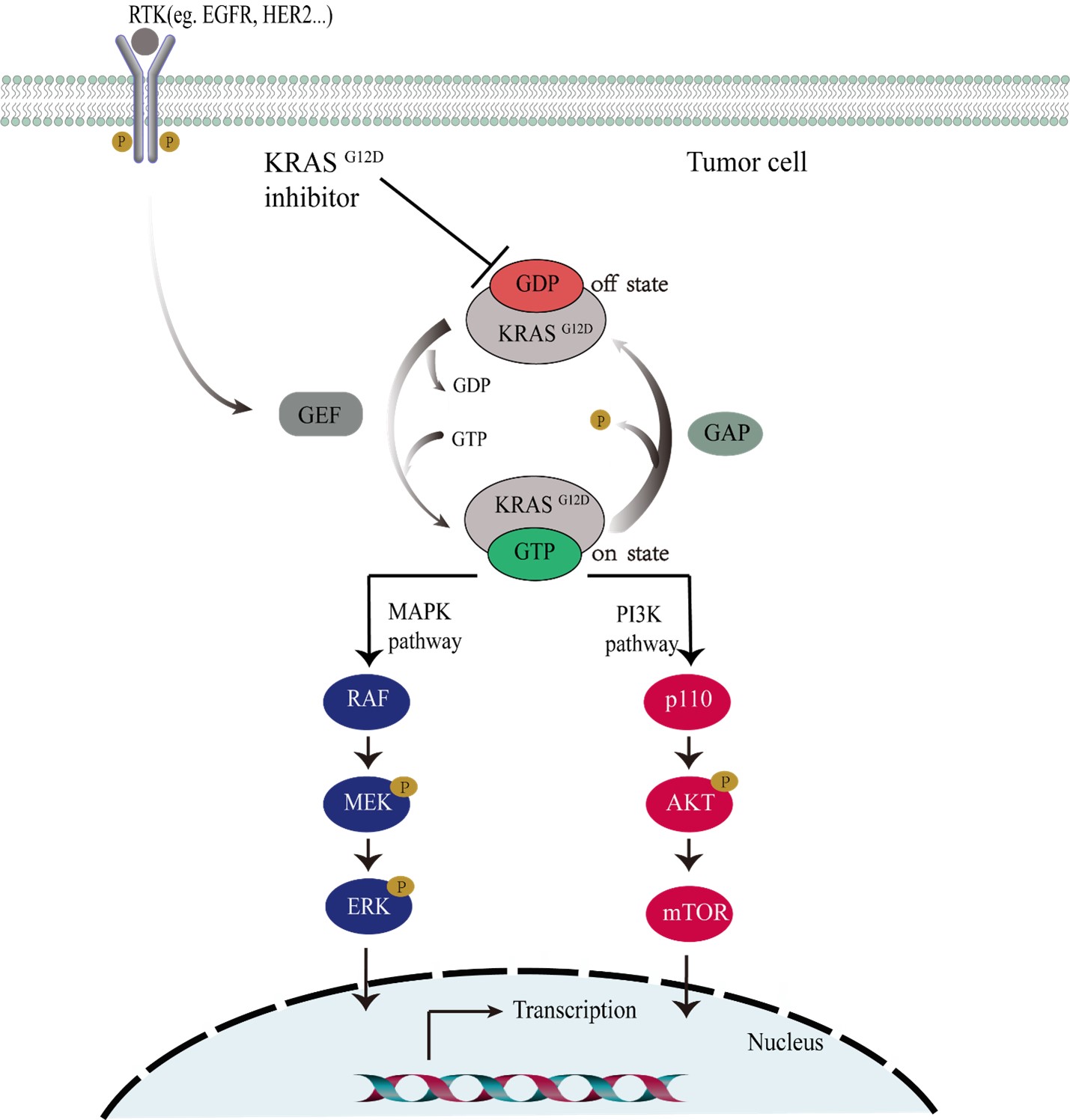
HBW-012
KRAS is one of the most frequently altered oncogenes, with mutations occurring in in 17%–25% of all malignancies. KRAS was once considered “undruggable” since its identification as a key driver of cancer 40 years ago. A therapeutic breakthrough came from the discovery of KRAS-G12C inhibitors, sotorasib (Lumakras) followed by adagrasib (Krazati), which act by forming a covalent bond with cysteine 12 of KRAS-G12C protein. Among KRAS variants, KRAS G12D mutation is indeed the most prent one, accounting for about 45% of all KRAS mutations. Its incidence is about 2.5 times higher than that of G12C mutation. Additionally, G12D is more common in cancer types that are refractory or with poor prognoses, especially in pancreatic cancer. The development of KRAS G12D inhibitors is of high therapeutic value but even more challenging since a KRAS G12D inhibitor will require a higher affinity for KRAS than a KRAS G12C inhibitor, which is allowed to bind covalently.
Hyperway has discovered a novel KRAS G12D inhibitor, HBW-012-D. Based on its preclinical profile, HBW-012-D is superior to competitors in its potency, efficacy, and pharmacokinetics properties. Importantly, its safety meets the criteria of clinical development. HBW-012-D is identified as a preclinical candidate compound (PCC) to be advanced to clinical studies in cancer patients soon.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan