Chengdu, China — Chengdu Hyperway Pharmaceutical Co., Ltd. (Hyperway Pharma) has achieved a pivotal milestone in innovative drug development. The Phase III clinical trial protocol for its first independently developed drug, HBW-3220 capsules, has successfully passed review by the Centre for Drug uation (CDE) of the National Medical Products Administration (NMPA). This milestone marks HBW-3220’s formal entry into the decisive Phase III clinical research stage and underscores Hyperway Pharma’s progress in bridging the critical link between innovative R&D and market value conversion.
Breakthrough in BTK Inhibitor Therapy
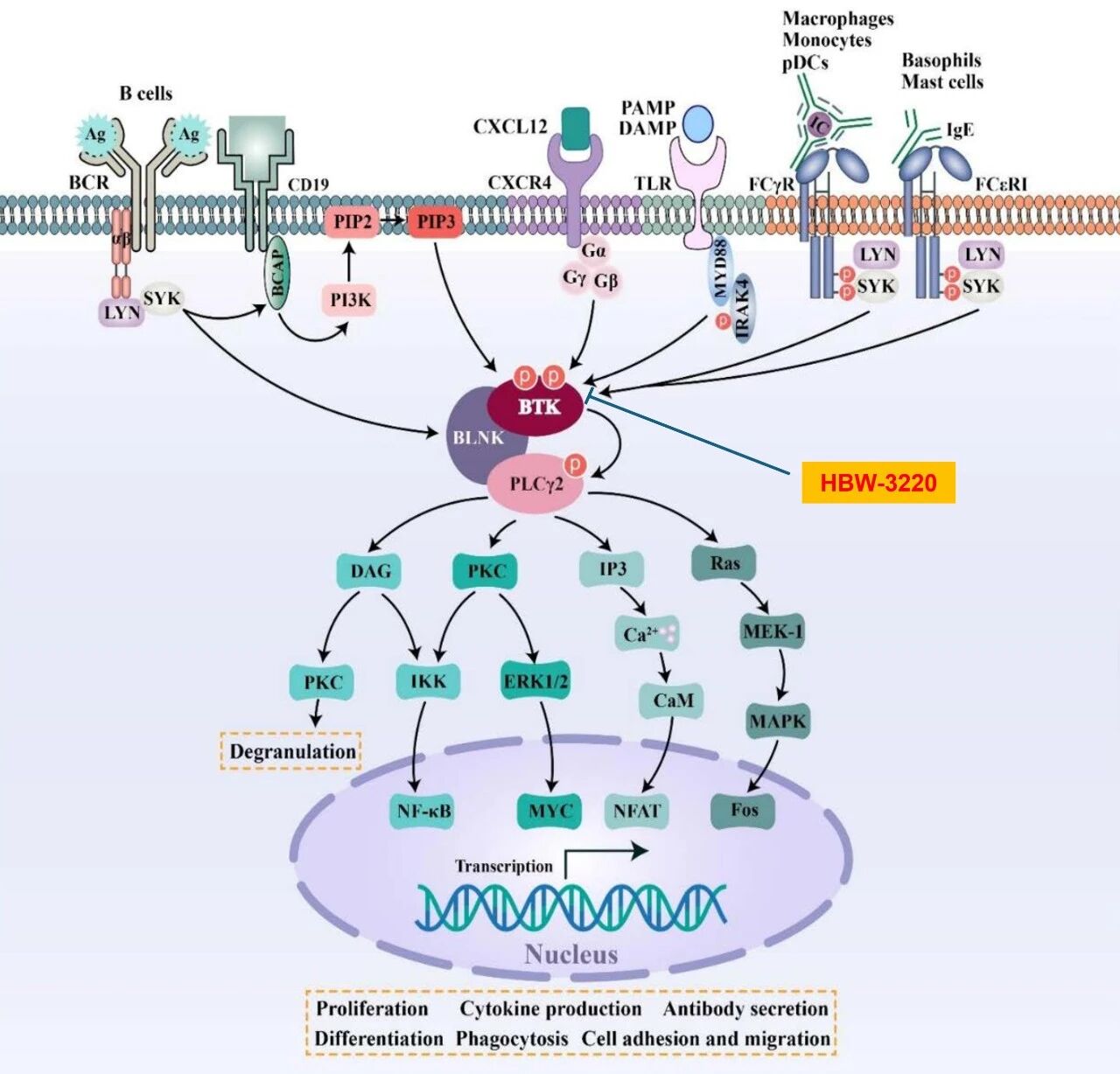
HBW-3220 capsules represent a core innovation independently developed by Hyperway Pharma. As a fourth-generation BTK inhibitor with a reversible, non-covalent molecular design, HBW-3220 directly addresses the longstanding challenge of drug resistance in BTK inhibitor therapy.
· Earlier generations of BTK inhibitors have been constrained by C481S mutation resistance.
· HBW-3220 achieves a cross-generation breakthrough, not only overcoming C481S resistance but also demonstrating superior inhibitory activity against multiple emerging mutations, including L528W and T474I.
This advancement offers enhanced therapeutic options for patients with B-cell lymphoma and positions HBW-3220 to fill a critical gap in the global BTK inhibitor market, valued at tens of billions of dollars.
Strong Clinical Foundation: Safety and Efficacy
Results from completed Phase I/II clinical trials highlight HBW-3220’s dual advantages:
· Excellent Safety Profile
o No dose-limiting toxicities (DLTs) were observed across all dose groups.
o The incidence of grade 3 or higher adverse reactions was significantly lower than that of the marketed third-generation BTK inhibitor Pirtobrutinib, providing strong assurance for long-term patient safety.
· Significant Therapeutic Efficacy
o Achieved near-complete objective response rates in CLL/SLL patients harboring C481S and T474I mutations.
o Demonstrated efficacy comparable to Pirtobrutinib in patients previously treated with BTK inhibitors, while uniquely addressing resistance mutations such as T474I, which Pirtobrutinib cannot resolve.
o Exhibited best-in-class (BIC) potential in BTK inhibitor–naïve patients, outperforming currently marketed therapies.
Expanding Market Potential
As the primary therapeutic category for treating B-cell lymphoma, the BTK inhibitor market continues to expand. Frost & Sullivan projects the global BTK inhibitor market will reach US$26.1 billion by 2030, underscoring substantial growth potential.
The value of HBW-3220 capsules extends beyond oncology. With clinical trial approval secured for an additional indication in glomerulonephritis, HBW-3220 demonstrates significant promise in autoimmune diseases. This enables Hyperway Pharma to pursue a dual-track strategy across oncology and autoimmune conditions, further expanding its market growth ceiling.
Accelerating Toward Industrialization
Hyperway Pharma has strengthened its clinical research team and is advancing the Phase III clinical trial of HBW-3220 capsules in B-cell lymphoma at full speed. This decisive step will accelerate the drug’s industrialization process, bringing new therapeutic hope to patients worldwide and reinforcing the company’s commitment to delivering innovative, life-changing treatments.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan