Chengdu Hyperway Pharmaceuticals Co., Ltd. (Hyperway Pharma) has unveiled groundbreaking clinical research results for its fourth-generation BTK inhibitor, HBW-3220 capsules. The data demonstrates an exceptional 100% objective response rate (ORR) in treating chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) patients with the C481S mutation, delivering renewed optimism to patients battling these conditions.
In the study, seven CLL/SLL patients carrying the C481S mutation experienced significant improvement following treatment. Remarkably, one patient with both C481S and T474I mutations achieved a partial response (PR) after completing eight treatment cycles, with tumor size reduced by over 50%. These findings emphasize HBW-3220's promising therapeutic potential, even for patients with the challenging T474I mutation, highlighting its differentiation in clinical practice.
Addressing Multi-Drug Resistance with HBW-3220
As the most rapidly advancing fourth-generation BTK inhibitor worldwide, HBW-3220 capsules exhibit exceptional potency against wild-type BTK, the C481S mutant, and other difficult-to-treat mutations, such as L528W and T474I. This breakthrough addresses the persistent challenge of multi-drug resistance observed with earlier generations of BTK inhibitors.
Compared to Pirtobrutinib (LOXO-305), the sole third-generation BTK inhibitor currently available, HBW-3220 has shown significant advantages in preclinical studies. These include superior in vitro inhibitory activity, enhanced in vivo efficacy, improved pharmacokinetics, and excellent clinical safety—positioning HBW-3220 as a potential game-changer with broad prospects.
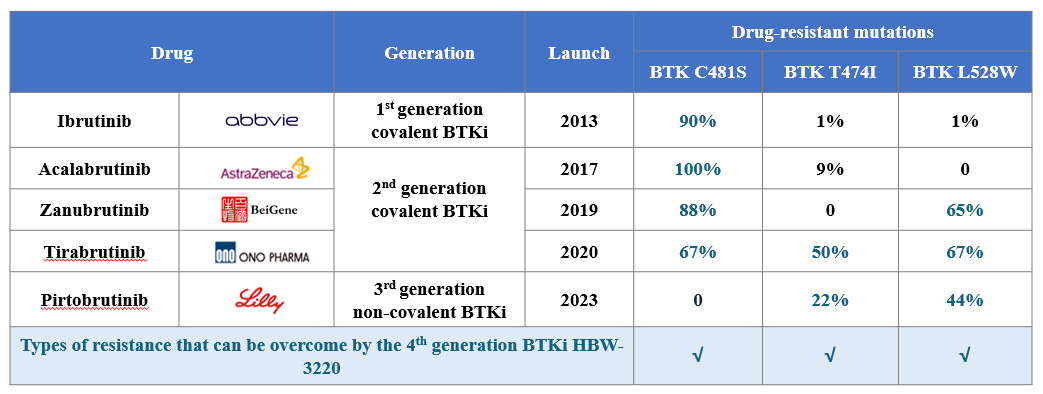
HBW-3220: Overcoming Multi-Mutant Drug Resistance Left Unaddressed by Earlier BTK Inhibitors
Strong Safety and Exceptional Efficacy Across Lymphomas
HBW-3220 demonstrated an impressive safety profile in clinical trials. Across all dosage groups, no dose-limiting toxicities (DLTs) were reported. Additionally, the rates of grade 3 or higher adverse events—such as reduced neutrophil count, hemoglobin, platelet count, and bleeding—were lower than those associated with Pirtobrutinib.
The drug also displayed remarkable potency in treating various types of lymphoma. For mantle cell lymphoma (MCL) patients with no prior BTK inhibitor treatment, the ORR reached 100%. Among CLL/SLL patients previously treated with irreversible BTK inhibitors and BCL2 inhibitors, HBW-3220’s ORR exceeded that of Pirtobrutinib. Its performance in marginal zone lymphoma (MZL) further underscores its clinical potential.
HBW-3220: A Transformative Force in the BTK Inhibitor Market
The achievements and positive data from HBW-3220 clinical studies position this fourth-generation BTK inhibitor as a transformative development in the field. With a projected follow-up BTK inhibitor market valued at tens of billions of USD, HBW-3220 shows immense promise. Comprehensive clinical data will be officially presented at the ASCO Annual Meeting in May.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan