On April 1, 2025, Chengdu Hyperway Pharmaceuticals (Hyperway Pharma) achieved a significant milestone with clinical trial approval from the National Medical Products Administration (NMPA) for HBW-012336—a groundbreaking oral KRAS G12D inhibitor. This innovative drug, designed to target KRAS G12D mutations in hard-to-treat cancers like pancreatic, colorectal, and non-small cell lung cancer, showcases high potency, selectivity, and tissue enrichment.
KRAS mutations drive approximately 30% of cancers, with KRAS G12D mutations accounting for 35% of these cases, making them a critical target for drug development. Despite its historical reputation asan “undruggable” protein, efforts in KRAS research have surged globally. With no KRAS G12D inhibitors currently approved, HBW-012336 stands as a promising candidate to address unmet medical needs in oncology. As Phase I clinical trials commence, Hyperway Pharma continues to advance its innovative pipeline, aiming to redefine possibilities in cancer treatment.Harnessing the Power of KRAS G12D Inhibition: HBW-012336
HBW-012336, Hyperway Pharma's oral KRAS G12D inhibitor, continues to showcase groundbreaking potential in oncology research. Here's why it's capturing global attention:
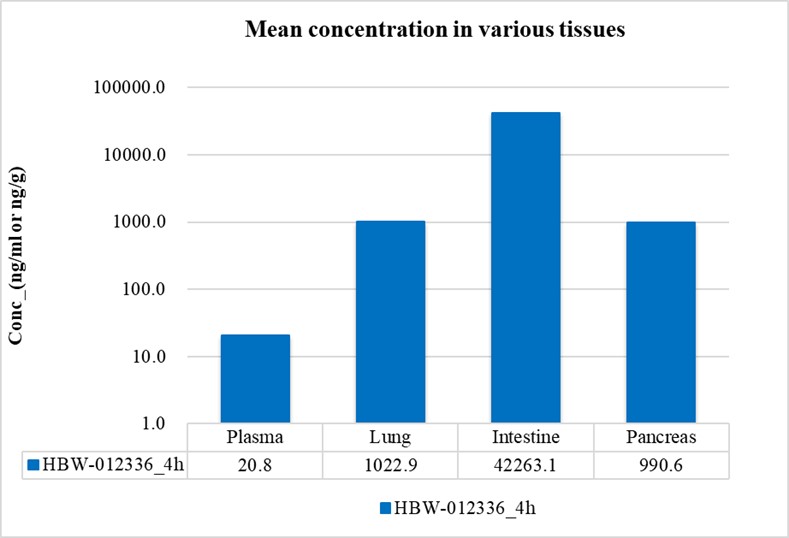
(1)Selective Potency: In vitro studies reveal HBW-012336's strong inhibitory effects against tumor cell lines carrying the G12D mutation, with exceptional selectivity over wild-type KRAS, HRAS, and NRAS.
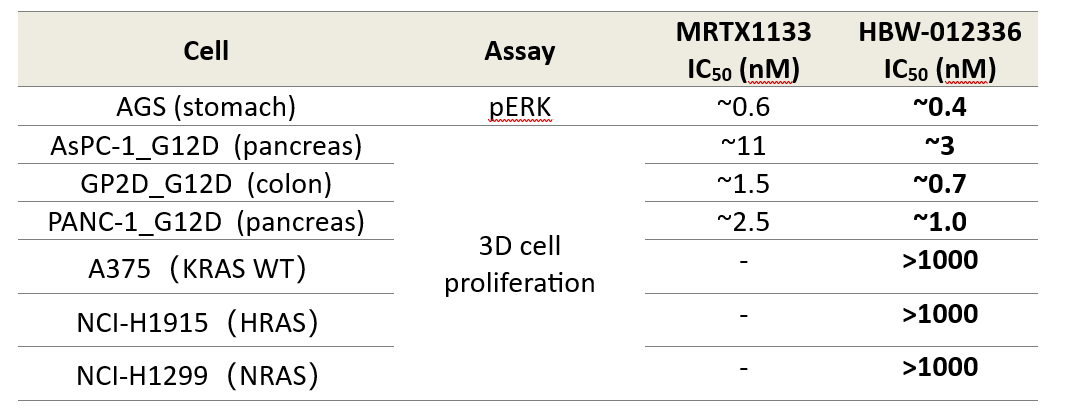
(2)Tissue Targeting: HBW-012336 demonstrates superior enrichment in tissues prone to high G12D mutation rates—pancreas, intestine, and lungs—ensuring precise therapeutic action.
(3) Impressive Efficacy: In vivo models highlight HBW-012336's ability to completely inhibit tumor growth or further reduce tumor volume. A notable synergy was observed in the LS513 human colon cancer model when combined with cetuximab, achieving a 22% tumor regression rate post-treatment.
(4) Safety Assurance: HBW-012336 exhibits a robust safety profile, with no significant impacts on cardiovascular, respiratory, or central nervous systems, and strong results from 28-day GLP toxicology studies in both rats and dogs.
As both a monotherapy and in combination with other cancer treatments, HBW-012336 demonstrates vast development and market potential. Its groundbreaking research findings have earned spots at the 2024 AACR and ASCO conferences, garnering interest from leading pharmaceutical companies worldwide.
Breaking New Ground in Pan-KRAS Therapeutics with HBW-016-K
Building on the success of HBW-012336, Hyperway Pharma is making strides in the field of Pan-KRAS inhibitors. Our innovative oral candidate, HBW-016-K, marks a significant milestone with its high potency, selectivity, and tissue enrichment properties. With comprehensive preclinical studies progressing, we’re on track to submit an IND application in 2025.
Breakthrough Highlights:
1. Broad Inhibitory Activity: HBW-016-K shows exceptional effects across KRAS mutations, including G12D, G12C, G12V, G12R, and G13D, supported by favorable pharmacokinetic profiles
2. Exceptional Efficacy: In vivo models demonstrate its ability to completely inhibit or shrink tumors, either as a monotherapy or in combination with a FAK inhibitor.
3. Strong Safety Profile: MTD and 14-day DRF studies in rats and dogs confirm a safety window of 20 to 40 folds, meeting requirements for further clinical development.
We’re also thrilled to share detailed findings at the coming 2025 AACR Annual Meeting in Chicago. Key details include:
Abstract Title: HBW-016-K, a novel orally available Pan-KRAS inhibitor targeting both "ON" and "OFF" states, is potent, selective, efficacious, safe, and high tissue-penetrant.
Session Information:
Session Category: Experimental and Molecular Therapeutics
Session Title: RAS Inhibitors
Session Time: 4/29/2025 9:00:00 AM - 12:00:00 PM
Location: Poster Section 21
Poster Board Number: 15
Published Abstract Number: 4380
Hyperway Pharma is proud to be at the forefront of KRAS-targeted therapy innovations, working tirelessly to address unmet needs in oncology. Follow our journey for more updates as we strive to make a difference in cancer treatment!

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan