Hyperway Pharma, officially known as Chengdu Hyperway Pharmaceuticals Co., Ltd., has recently completed the phase I clinical study of their independently developed Nav1.8 inhibitor, HBW-004285. The clinical data indicate that, when compared to VX-548(Journavx)—the first Nav1.8 inhibitor to reach the market—HBW-004285 boasts a higher safety profile with no adverse reactions above Grade 1. Additionally, HBW-004285 demonstrates a faster onset of action, superior pharmacokinetic characteristics, and no gender differences, positioning it as a strong contender to surpass VX-548 and become a Best-In-Class medicine.
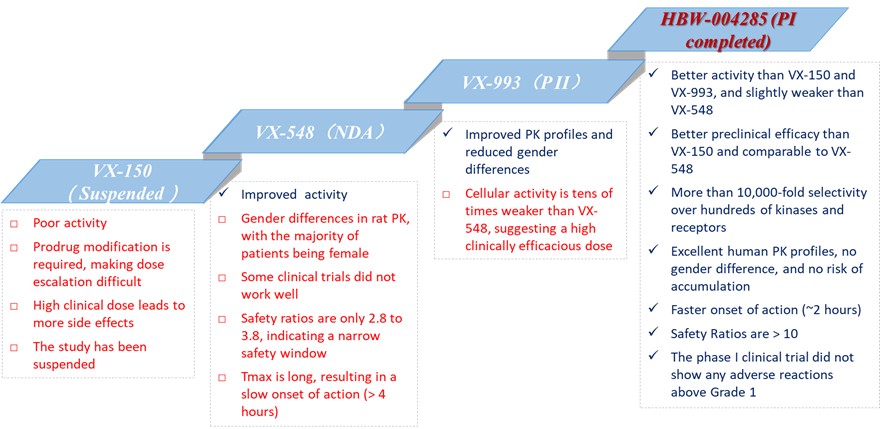
Figure 1: HBW-004285 Tablet V.S. Vertex Compounds
Nav1.8 is a voltage-gated sodium channel primarily expressed in sensory neurons of the peripheral nervous system. It plays a key role in transmitting pain signals without causing opioid-like addiction, making it highly sought after as a pivotal target in analgesic drug development.
VX-548 (Journavx), developed by Vertex Pharmaceuticals, is the first Nav1.8 inhibitor approved by the U.S. Food and Drug Administration (FDA) on January 30, 2025. VX-548 is also the first non-addictive drug for acute pain treatment introduced in over 20 years—a landmark achievement. Vertex's clinical success with VX-548 led to a 13% surge in their stock price in a single day, pushing their market value past $100 billion. Leerink Partners analysts predict that by 2030, annual sales of VX-548 could reach $5.1 billion, underscoring the significant market potential and clinical importance of VX-548 and other Nav1.8 inhibitors.
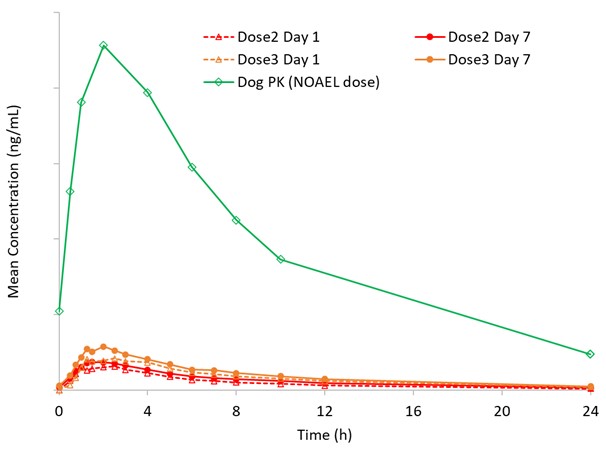
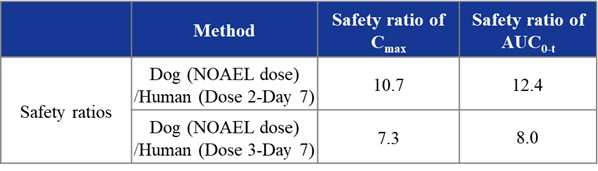
Figure 2: HBW-004285 PK Curves and Safety Ratios
HBW-004285 is a pivotal analgesic drug in Hyperway Pharma’s pipeline. When compared to VX-548, HBW-004285 has showcased exceptional advantages in preclinical head-to-head comparisons, such as a superior pharmacokinetic profile, faster and longer-lasting analgesic effects, and higher safety. These preclinical benefits have been validated in the phase I clinical trials.
Higher Safety: None of the subjects experienced adverse reactions above Grade 1. Only some subjects had Grade 1 adverse reactions (including some subjects in the placebo group) and showed no dose dependence. Additionally, a reported 28-day GLP toxicity test in monkeys showed that VX-548 had safety ratios of 2.8 (AUC(0-t)) and 3.8 (Cmax) at the clinically efficacious dose, suggesting a narrow safety window. Conversely, a 28-day GLP toxicology study in dogs revealed that HBW-004285 tablets had safety ratios more than 10 times higher, indicating significantly better clinical safety compared to VX-548.
Faster Onset of Action: Phase I clinical studies demonstrated that HBW-004285 tablets reached Tmax at 1.5-2 hours post-administration. Rapid onset of pain relief is especially critical for both acute and chronic pain management. In comparison, the average human Tmax of VX-548 is 6 hours, with its active metabolite reaching Tmax at 12 hours according to phase II clinical results.
Superior Pharmacokinetic Characteristics: HBW-004285 tablets exhibited excellent clinical dose linearity, with no accumulation tendency and no significant gender differences. In the MAD (Multiple Ascending Dose) study, the exposure (AUC) of HBW-004285 tablets at Dose 2 was approximately twice that of Dose 1, and at Dose 3, it was 1.5 times that of Dose 2. The exposure on the 7th day was 1.25-1.37 times that of the first day, indicating no accumulation tendency.
Promising Analgesic Trends: Preliminary data suggest that HBW-004285 may have a superior analgesic effect compared to VX-548. Blood concentrations of HBW-004285 at all dose groups exceeded Adjusted IC90 levels (converted according to the free drug concentration). Based on current clinical data, it is anticipated that HBW-004285 tablets can produce significant analgesic effects at Dose 2. Additionally, Figure 3 analysis indicates that HBW-004285 may offer more effective analgesia than VX-548 in humans based on Cmax/Adjusted IC50 or IC90 value analysis.
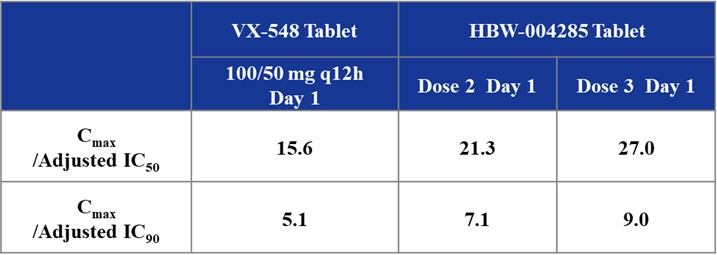
Figure 3: The Comparison of Drug Concentrations in Human Blood with Adjusted IC50 and Adjusted IC90
Overall, Hyperway Pharma's Nav1.8 inhibitor, HBW-004285 tablets, have demonstrated good safety, tolerability, pharmacokinetic characteristics, and preliminary analgesic effects in phase I clinical studies. With the potential to surpass VX-548 and become a Best-In-Class medicine, HBW-004285 shows significant market development prospects. Hyperway Pharma is committed to rapidly advancing the research and development of the HBW-004285 project and is expected to complete the phase II clinical study and initiate the phase III clinical study in 2025.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan