KRAS is a major driver of cancer development and progression. Patients with KRAS mutations often have a worse prognosis compared to those without. Approximately 30% of cancer patients harbor KRAS mutations, with a particularly high prence in pancreatic cancer (90%), colorectal cancer (40%), and non-small cell lung cancer (30%). Additionally, KRAS mutations are found in a significant percentage of endometrial, bile duct, ovarian, bladder, liver, and breast cancers.
In 2021, Lumakras, the world's first approved KRAS inhibitor, overturned the long-held belief that KRAS was an undruggable target. The field of KRAS inhibitor development is rapidly expanding, with over 60 inhibitors currently being uated in clinical trials. These inhibitors include those targeting specific KRAS mutations, such as G12C and G12D, and pan-KRAS inhibitors targeting various KRAS mutations. Despite potential market challenges for Lumakras, GlobalData forecasts that the global market for KRAS-targeted drugs will surpass $4 billion by 2029. This indicates a significant market potential and sustained development opportunities for KRAS inhibitors in the future.
Through innovative drug design efforts, Hyperway Pharma has identified two types of KRAS inhibitors: HBW-016-K, a pan-KRAS inhibitor, and HBW-012-D &-E, KRAS G12D inhibitors. These oral inhibitors demonstrate high potency, selectivity, and tissue enrichment, and are currently undergoing preclinical uation. IND applications are planned for 2024-2025.
Pan-KRAS inhibitor HBW-016-K
Pan-KRAS inhibitors can target multiple KRAS mutations, expanding their potential patient population. They address the common issue of resistance associated with single mutant-selective inhibitors and can be combined with other targeted therapies (e.g., SOS1, SHP2, EGFR) for enhanced therapeutic effects. With a market value estimated to be eight times that of KRAS G12C inhibitors, pan-KRAS inhibitors are considered potential blockbusters. The development of pan-KRAS inhibitors is often called the ultimate battle in KRAS research.
Preclinical studies have shown that HBW-016-K, a Pan-KRAS inhibitor developed by Hyperway Pharma, has significant advantages:
(1) HBW-016-K demonstrated potent inhibition of pERK in AGS gastric cancer cells and HPAC pancreatic cancer cells, with IC50 values of approximately 2 nM. Additionally, HBW-016-K strongly inhibited cell proliferation in various KRAS-mutant cancer cell lines, including NCI H358 non-small cell lung cancer, GP2D colon cancer, KP-4 pancreatic cancer, and RKN ovarian cancer, demonstrating its broad-spectrum activity against different KRAS mutations.
(2) While RMC-6236, a pan-RAS inhibitor from Revolution, only targets the active state of KRAS G12D, HBW-016-K can target both the active and inactive states. This unique property suggests that HBW-016-K may have a superior therapeutic effect.
(3) Kinase profile screening experiments show that HBW-016-K has high selectivity, and 14-day toxicology studies in rats show that HBW-016-K has a nearly 17-fold safety window at least, which fully meets the needs of clinical development.
(4) HBW-016-K demonstrates exceptionally high tissue enrichment, particularly in lung, intestinal, and pancreatic tissues. The concentration of HBW-016-K in these tissues is significantly higher than in plasma, making it an ideal candidate for the treatment of lung, colorectal, and pancreatic cancers.
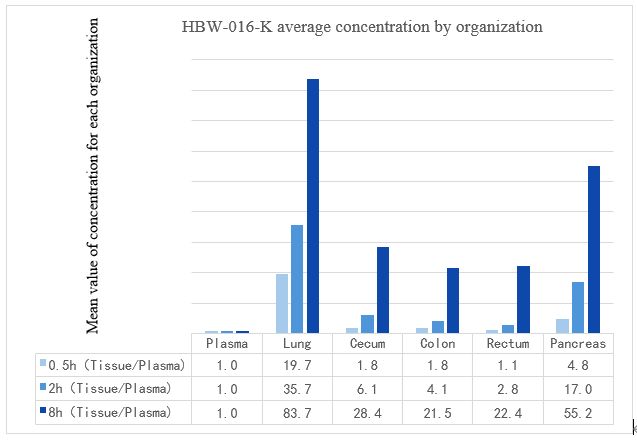
Figure 1: Distribution of HBW-016-K in various tissues
As shown in Figure 1, HBW-016-K demonstrated significantly higher concentrations in target tissues, such as the lung, intestine, and pancreas, compared to plasma levels. In particular, lung and pancreatic tissues had concentrations approximately 80 and 50 times higher than plasma, respectively. This suggests that HBW-016-K is strongly enriched in these organs, making it a promising candidate for the treatment of lung cancer, colorectal cancer, and pancreatic cancer.
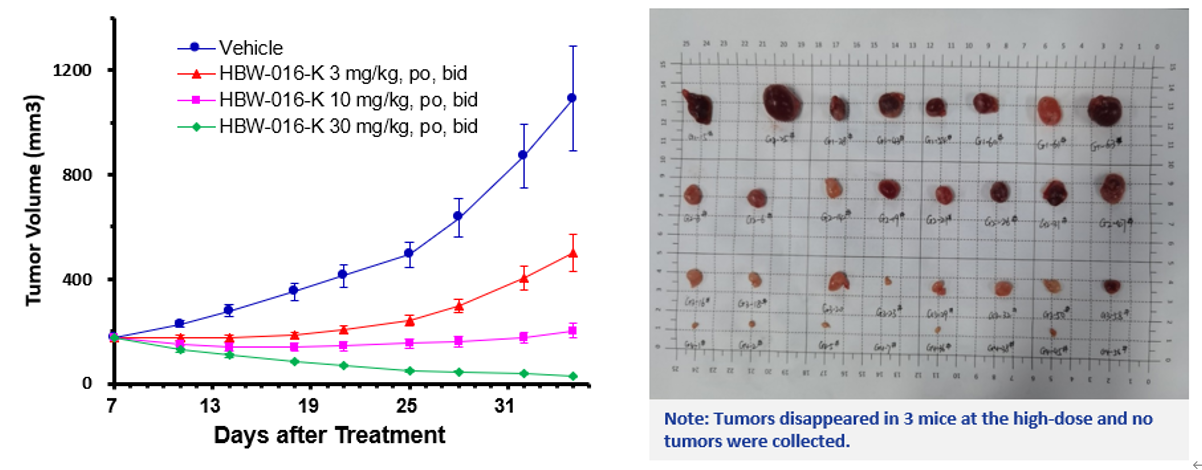
Figure 2: In vivo efficacy assay of HBW-016-K in RKN G12V mice
As can be seen in Figure 2, in the RKN G12V (ovarian cancer) mouse model, administered for 28 days, HBW-016-K showed good tumor inhibition at a dose of 3 mg/kg BID, and tumor shrinkage of 84.1% at a high dose of 30 mg/kg BID (compared with the tumor size at grouping), which showed excellent anti-tumor effects; Moreover, HBW-016-K in the RKN G12V mouse model showed a good dose-dependent relationship, and there was no abnormality in the state of mice during the entire oral administration.
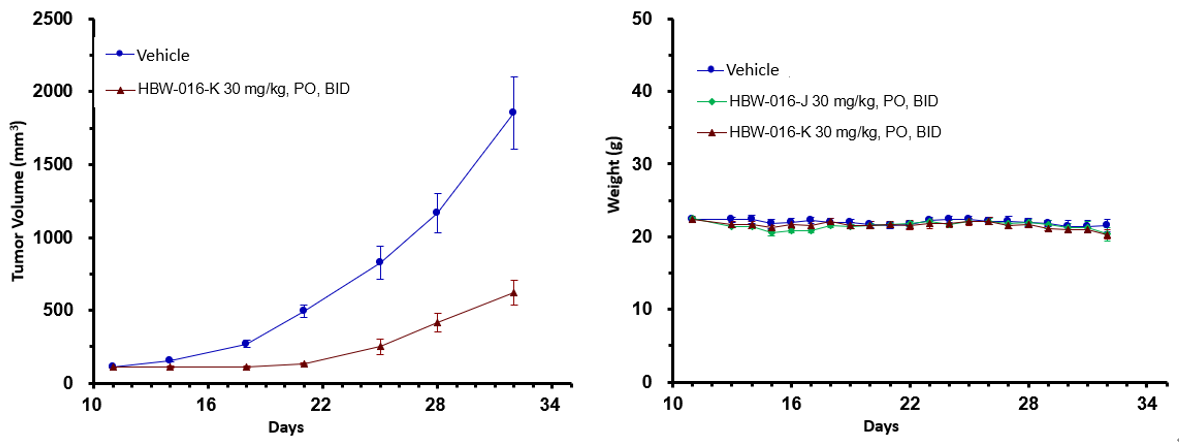
Figure 3: In vivo efficacy assay of HBW-016-K in KP-4 G12D mice
As shown in Figure 3, in the in-vivo pharmacodynamic model of KP-4 G12D (pancreatic cancer) mice, administered orally for 21 days, HBW-016-K exhibited good tumor suppression with a TGI of 70.7% and was well tolerated.
KRAS G12D Inhibitors HBW-012-D &-E
KRAS G12D is the most prent KRAS mutation, occurring approximately 2.5 times more frequently than the G12C mutation. It is often found in challenging-to-treat cancers, such as pancreatic and colorectal cancer, highlighting a significant unmet clinical need.
In April 2024, Hyperway Pharma presented preclinical research results on HBW-012-D and HBW-012-E, two highly active, selective, and tissue-enriched oral KRAS G12D inhibitors. The findings were showcased at the annual meeting of the American Association for Cancer Research (AACR), attracting significant interest from pharmaceutical companies worldwide.
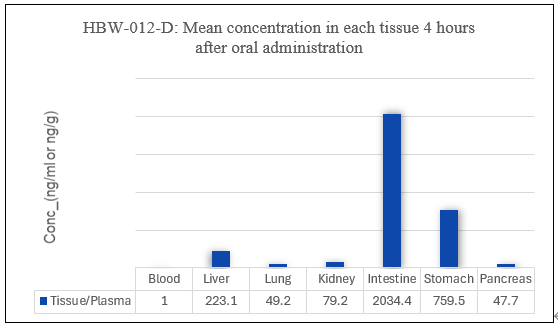
![]()
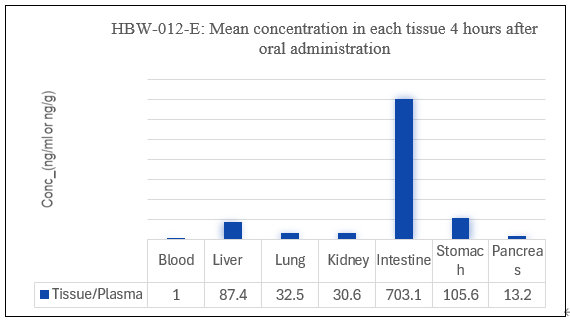
Figure 4: Mean concentration in each tissue after oral administration of HBW-012-D/E
As shown in Figure 4, tissue distribution studies in rats demonstrated that the concentration of HBW-012-D and HBW-012-E in target organs, such as the colon, pancreas, and lungs, was significantly higher than plasma levels after 4 hours of oral administration. This indicates a clear tissue enrichment, suggesting that these drugs are promising candidates for the treatment of colorectal cancer, pancreatic cancer, and lung cancer.
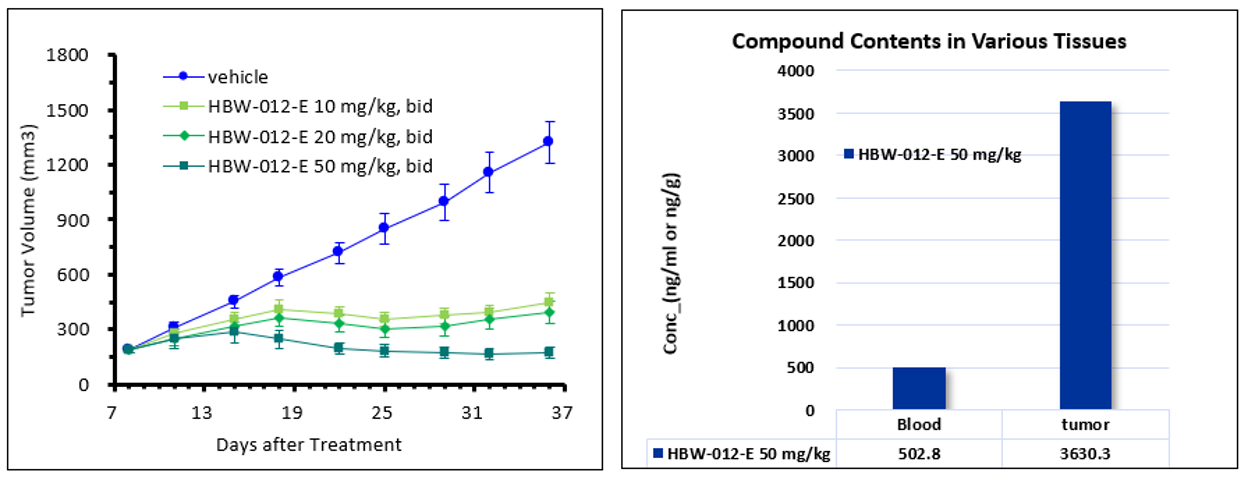
Figure 5: In vivo pharmacodynamic study of HBW-012-E in GP2D mice
As can be seen in Figure 5: In GP2D (colon cancer) mice, oral administration of HBW-012-E in the 10 and 20 mg/kg dose groups resulted in tumor growth inhibition (TGI) of 77% and 82%, respectively; in 50 mg/kg dose group resulted in a further reduction in tumor volume by about 10%, showing a dose-dependent effect.
Tissue distribution studies revealed that the concentration of HBW-012-E in tumor tissues was significantly higher than plasma levels, reaching more than 7-fold at a dose of 50 mg/kg twice daily. No abnormality was observed in any animal in all three drug groups during 28 consecutive days of treatment, demonstrating a good safety profile.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan