In the latest clinical study, a patient with primary central nervous system lymphoma (PCNSL) who had previously failed treatment with brain-penetrating irreversible BTK inhibitors experienced a rapid reduction in brain tumor size after taking HBW-3210 capsules, and the tumor vanished completely after 6 cycles of treatment. HBW-3210 is an innovative drug developed independently by Chengdu Hyperway Pharmaceutical Co., Ltd. (referred to as "Hyperway Pharma"). The remarkable efficacy of HBW-3210 capsules validates their potential and showcases Hyperway Pharma's strength in innovative drug research and development.
Li Wenbin, director of the Comprehensive Cancer Treatment Center of Beijing Tiantan Hospital affiliated with Capital Medical University, the lead PI of the clinical study for this project, commented: Based on the preliminary clinical trial data, HBW-3210 capsules have demonstrated a favorable safety profile and good tolerability. Additionally, several patients have experienced tumor shrinkage, indicating promising efficacy. The trial is progressing well, and we anticipate HBW-3210 will benefit more patients with relapsed/refractory PCNSL.
Introuction to PCNSL
Primary central nervous system lymphoma (PCNSL) is the most challenging type of non-Hodgkin's lymphoma originating in the brain, soft tissues, spinal cord, or eye, with more than 95% of the histopathologic type being diffuse large B-cell lymphoma (DLBCL). PCNSL is highly aggressive and fast-progressing, and compared with other types of lymphoma, patients have short survival and poor prognosis. Prevention and treatment options are currently limited, and a significant unmet clinical need exists. Due to its location in the central nervous system and the presence of the blood-brain barrier, PCNSL is difficult to treat with most drugs, that are generally not brain penetrating. The key to effective PCNSL chemotherapy is selecting drugs that can penetrate the blood-brain barrier. High-dose methotrexate is a preferred option and can be combined with high-dose cytarabine, temozolomide, rituximab, or other agents to enhance therapeutic efficacy. Patients who have achieved remission with first-line treatment can undergo consolidation therapy, including autologous hematopoietic stem cell transplantation, cytarabine ± etoposide or whole-brain radiotherapy, etc. Patients with relapsed or refractory PCNSL can choose their treatment based on their initial treatment plan and the timing of relapse. As there is no standard treatment, participating in clinical trials of promising new drugs is recommended.
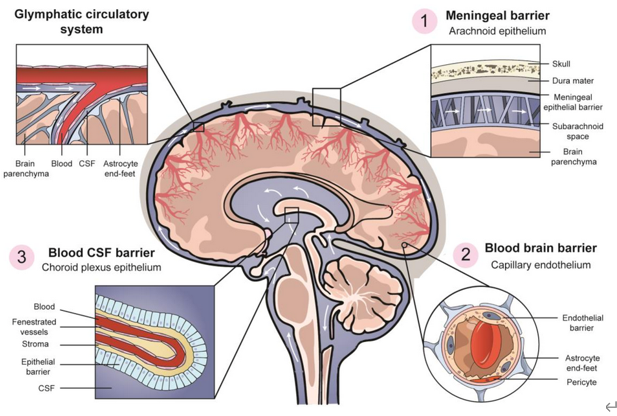
(Image via Elsevier)
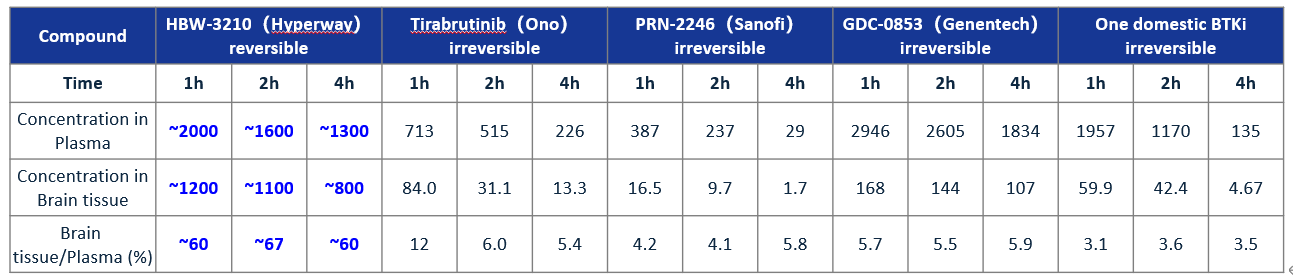
Currently, Tirabrutinib, developed by Ono Pharmaceuticals, is the only approved treatment for relapsed or refractory PCNSL. Tirabrutinib is 1st generation of irreversible BTK inhibitors, with a brain penetration rate of about 10%, and after taking it for some time, the patient will develop drug resistance due to the occurrence of the C481S mutation, which leads to disease progression. Currently, there are no approved brain-penetrating BTK inhibitors available in China that can overcome drug resistance. HBW-3210 capsule, developed by Hyperway Pharma, is a 3rd generation reversible BTK inhibitor. Its preclinical study has shown that: 1) HBW-3210 can overcome the resistance due to the C481S mutation in BTK. It exhibits a strong inhibitory effect on both wild-type and C481S-mutant BTK kinase, suggesting that HBW-3210 could be effective for patients regardless of prior BTK inhibitor use. 2) the brain permeability of HBW-3210 was greater than 60%, much higher than that of current drugs for the treatment of PCNSL and other related brain tumors; 3) in the animal models with intracranial follicular lymphoma (DOHH2), a head-to-head comparison showed that HBW-3210 demonstrated significantly better efficacy compared to Tirabrutinib.
Table 1: Summary of comparative studies on brain permeability in rats
Progress of clinical research on HBW-3210 capsule
HBW-3210 capsule is currently undergoing a phase I/II clinical study in patients with B-cell lymphoma, including primary central nervous system lymphoma (PCNSL) or secondary CNS lymphoma (SCNSL), chronic lymphocytic leukemia/small cell lymphoma (CLL/SLL), mantle cell lymphoma (MCL), follicular lymphoma (FL), marginal zone lymphoma (MZL), Waldenström's macroglobulinemia (WM)/lymphoplasmacytic lymphoma (LPL) and diffuse large B-cell lymphoma (DLBCL).
Patients in the enrolled relapsed/refractory PCNSL cohort had previously taken irreversible BTK inhibitors. The available clinical data show that HBW-3210 capsules have an excellent human pharmacokinetic profile and a favorable safety profile with no dose-limiting toxicity (DLT) events in all patients. In an ongoing dose-escalation trial, a patient with refractory PCNSL who had previously taken a brain-penetrating irreversible BTK inhibitor received the second escalation dose of HBW-3210 capsules. The patient achieved partial remission with significant brain tumor shrinkage within 2 cycles, and the tumors continued to shrink over 4 cycles. After 6 cycles, the tumors were no longer visible on imaging. Another PCNSL patient who had previously taken the brain-penetrating and irreversible BTK inhibitor also showed significant shrinkage of brain tumor even after 1 cycle of the HBW-3210 capsule!
In addition, analysis of drug concentration in the cerebrospinal fluid of existing patients showed that the cerebrospinal fluid (CSF)/plasma concentration ratio (blood-brain barrier permeability) of HBW-3210, after corrected by plasma protein binding, was approximately 63%, which was higher than the blood-brain barrier permeability of all other known irreversible BTK inhibitors, suggesting that HBW-3210 capsule will have a better efficacy on patients with central nervous system lymphoma. Hyperway Pharma will make every effort to promote the clinical research of this project and strive to benefit more patients as soon as possible.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan