On January 10, 2025, HBW-012336, a Class 1 innovative drug independently developed by Chengdu Hyperway Pharmaceuticals Co., Ltd. (hereinafter referred to as "Hyperway Pharma"), officially submitted an Investigational New Drug (IND) application to CDE of the National Medical Products Administration (NMPA). This marks another breakthrough in Hyperway Pharma's R&D pipeline.
HBW-012336 is a KRAS G12D inhibitor designed to treat multiple tumors with KRAS G12D mutations. Preclinical in vitro and in vivo data demonstrate that HBW-012336 exhibits high potency, selectivity, tissue enrichment, and strong anti-tumor efficacy. The study results have been selected for presentation at the 2024 American Association for Cancer Research (AACR) Annual Meeting and the American Society of Clinical Oncology (ASCO) conferences, attracting significant attention from renowned pharmaceutical companies worldwide. For more details, please refer to Hyperway's presentation at the 2024 AACR entitled: “HBW-012-D and HBW-012-E are Novel, Potent, Selective, Safe, and Bioavailable KRAS G12D Inhibitors with Superior Anti-Tumor Efficacy in Mice”, and 2024 ASCO entitled: “Preclinical characterization of HBW-012-E, a novel, potent, selective, safe, and orally active KRAS G12D inhibitor with superior pharmacokinetic (PK) properties and anti-tumor efficacy”.
The KRAS G12D mutation is the most common KRAS mutation, accounting for about 35% of all KRAS mutations. It is frequently found in difficult-to-treat cancers like pancreatic and colorectal cancers, which present an urgent clinical need. Despite the challenges, no KRAS G12D inhibitor has been approved worldwide, and current progress is mostly in Phase I clinical trials.
HBW-012336, an oral KRAS G12D inhibitor, improves oral bioavailability and strongly inhibits tumor cells with G12D mutations. It shows exceptional tissue enrichment in the pancreas, intestine, and lungs, which often develop tumors with G12D mutations, and is expected to offer significant therapeutic benefits for these cancers.
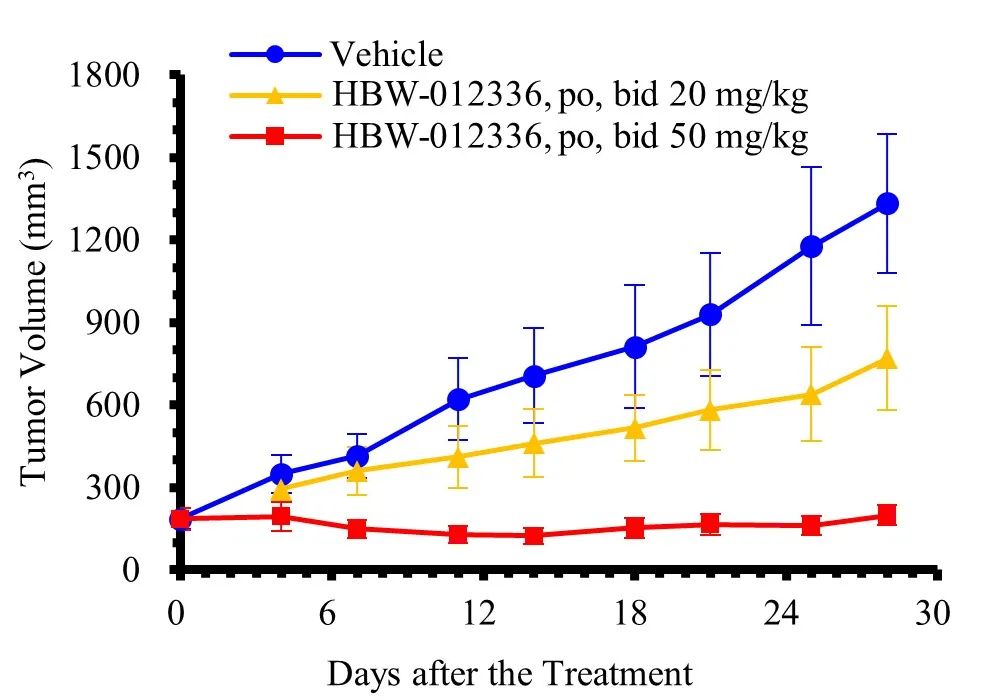
Preclinical studies have shown that HBW-012336 does not significantly affect the cardiovascular, respiratory, and central nervous systems, nor does it exhibit genotoxicity. It has demonstrated sufficient safety in toxicological studies of both monotherapy and repeated dosing. In various mouse xenograft models, HBW-012336 exhibited significant anti-tumor efficacy, with tumor inhibition effects notably surpassing those of MRTX1133, a known competitor compound, at the same dose. Additionally, in the LS513 xenograft model of human colon cancer cells, HBW-012336 combined with Cetuximab showed clear synergistic effects. Overall, HBW-012336 can be used as a single agent to treat tumors carrying KRAS G12D mutations or in combination with other anti-tumor drugs to enhance anti-tumor effects, presenting broad development and market prospects.
GP2d xenograft model of human colon cancer cell (oral)
In addition to developing KRAS G12D inhibitors, Hyperway Pharma is also advancing an oral Pan-KRAS inhibitor R&D pipeline and has identified HBW-016-K as a promising candidate compound. HBW-016-K exhibits strong inhibitory effects on KRAS G12D, G12V, G12R, G13D, and other mutations, and has demonstrated favorable pharmacokinetic characteristics in preclinical studies. In in vivo efficacy model studies with KRAS G12V mice, oral administration of HBW-016-K significantly regressed tumors. The project is currently in preclinical studies and is expected to be submitted for IND in 2025.
RKN xenograft model of human ovarian cancer cell (oral)
(Note: Tumors disappeared in 3 mice at 30 mg/kg)


Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan