On July 30, 2024, Vertex announced that the U.S. Food and Drug Administration (FDA) had accepted its New Drug Application (NDA) submission for its newly developed analgesic drug VX-548 (Suzetragine) to treat moderate-to-severe acute pain and granted priority review designation. VX-548, a Nav1.8 inhibitor, could revolutionize pain management. Unlike commonly prescribed opioid analgesics, it is free from the potential for addiction. If approved, it would be the first non-opioid, non-addictive pain treatment in over two decades. This breakthrough has significantly boosted Vertex's market capitalization, reaching $50 billion in the past year, and highlighting VX-548's promising potential.
Pain is one of the most common clinical symptoms, and analgesics as the primary method for pain management, have a huge and urgent market demand. According to the data of Wisdom Research Consulting, the market size of the global analgesics industry will be about $91.14 billion in 2022, and the market size of China's analgesics industry will be about 122.60 billion yuan. Within the next decade, China is poised to become the world's largest and most rapidly expanding pain management market. Consequently, the development of innovative analgesics that can challenge the dominance of NSAIDs and opioid analgesics has emerged as a critical scientific endeavor demanding collaborative exploration. The Nav1.8 inhibitor represents one such groundbreaking avenue.
Nav1.8 plays a key role in nociceptive signaling in the peripheral nervous system, and Nav1.8 inhibitors block the transmission of nociceptive signals from the peripheral nervous system to the central nervous system. Selective Nav1.8 inhibitors to pain-sensing neurons provide a significant advantage over non-selective Nav inhibitors. This selectivity reduces the likelihood of adverse effects and avoids the addiction risks associated with opioids. Moreover, their minimal involvement in central nervous system functions ensures minimal impact on essential cognitive and motor abilities. These factors make Nav1.8 inhibitors a promising target for developing novel analgesics.
The development of HBW-004285, an analgesic drug from Hyperway Pharma
Hyperway Pharma's focus on pain management has led to a robust pipeline of analgesic drug candidates. Among these, the Nav1.8 inhibitor HBW-004285 has garnered significant attention due to its exceptional properties. With its strong in vitro potency, sustained analgesic effects in animals, selectivity, and safety, HBW-004285 stands out as a leading Nav1.8 inhibitor in the global development landscape. The successful completion of Phase I clinical trials underscores its potential as a promising analgesic drug.
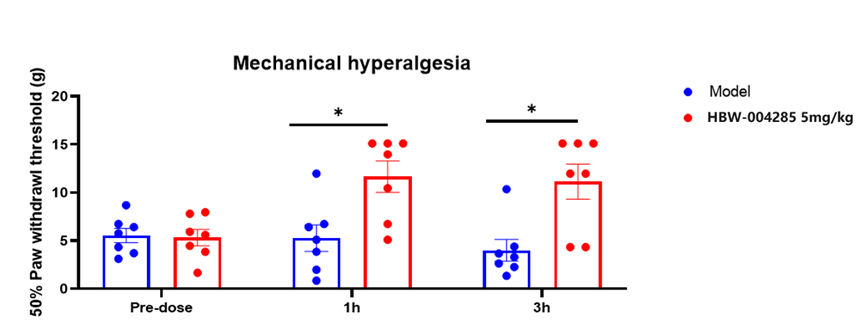
Figure 1:Rat Diabetic Peripheral Neuropathy Pain Model Study of HBW-004285
Preclinical studies showed that HBW-004285 demonstrated good analgesic effects and long-lasting analgesic duration in the complete Fuchs' adjuvant-induced rat foot pain model (chronic pain), SNL rat neuropathic pain model (chronic pain), rat plantar incision pain model (acute pain), and canine incision pain model (acute pain). In the rat diabetic peripheral neuropathy pain model (chronic pain), HBW-004285 also demonstrated good analgesic effects at a dose of 5 mg/kg (Figure 1), suggesting that it also has significant therapeutic potential for diabetic peripheral neuropathy pain (the American Diabetes Association predicts that the global diabetes patient population will increase to 366 million by 2030, and that prence of diabetes is driving the rapid growth of the diabetic neuropathy market).
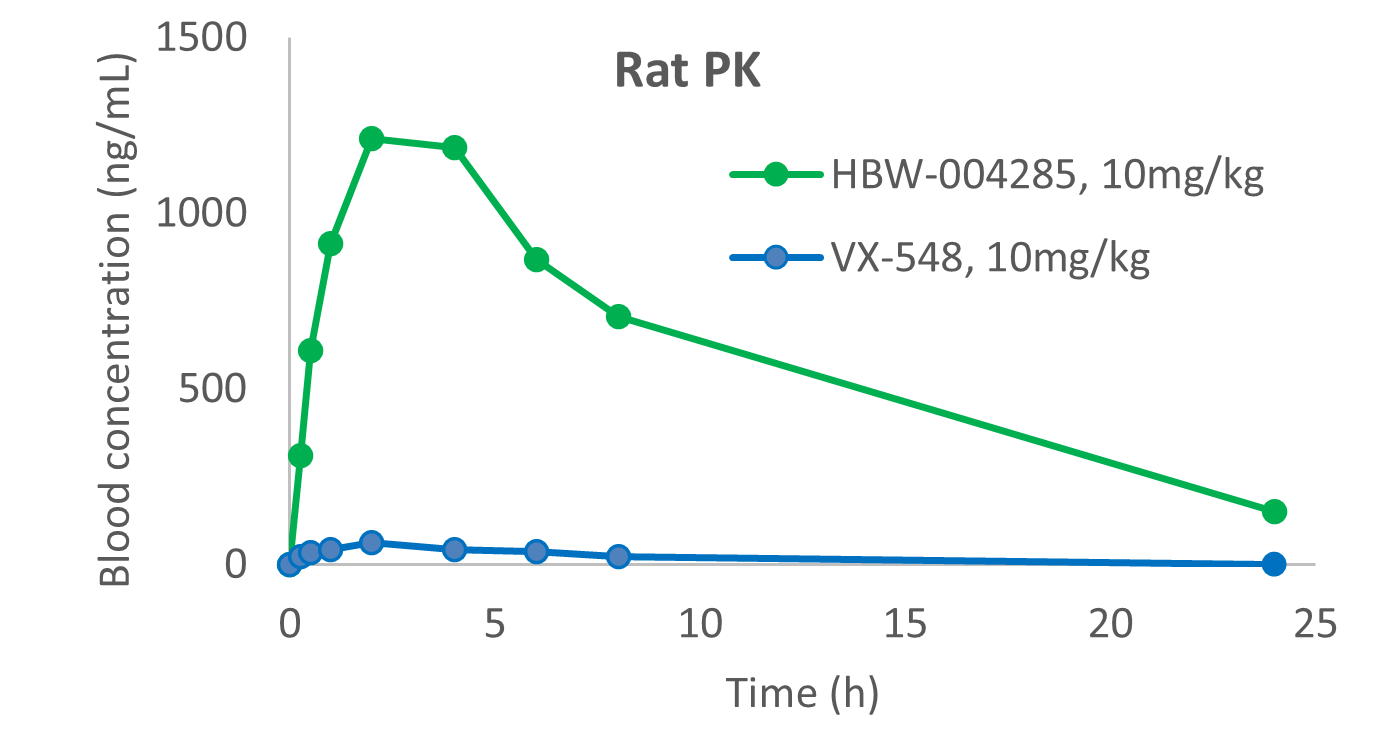
Figure 2: Comparative study on preclinical PK and safety of HBW-004285 and VX-548
As demonstrated in Figure 2, a preclinical head-to-head comparative pharmacokinetic and safety study showed that HBW-004285 had significantly higher exposure in rats compared to VX-548 when administered orally at a dose of 10 mg/kg.
The Phase I clinical study demonstrated the favorable safety profile of HBW-004285. Additionally, the results indicated a promising trend toward analgesic effects in humans, rapid onset of action, and suitability for both acute and chronic pain management.
(1) HBW-004285 capsules demonstrated a favorable safety profile in healthy subjects, regardless of whether they were taken as a single dose or continuously on an empty stomach. The incidence of adverse events was comparable to the placebo group.
(2) HBW-004285 was absorbed rapidly in the body after single and multiple administrations, and the exposure increased with dose dependence;
(3) Pharmacodynamic studies in humans have revealed that a significant analgesic trend can be observed in healthy subjects in some dose groups, especially in the pressure pain test. It is believed to have a more significant analgesic effect on patients.
Moreover, to further improve the bioavailability in the human body and meet the drug needs of more patient groups, in addition to capsules, Hyperway Pharma has also developed tablets (IND has been filed, tablets will be used in phase II), injections, and other drug formulations with better absorption and faster onset of action for HBW-004285. The exposure of newly developed tablets in dogs is about twice that of HBW-004285 capsules. The clinical dosage of tablets is expected to be lower than that of capsules, and the safety will be even better. The injectable formulation of HBW-004285 is fully compliant with clinical use standards and is currently being prioritized for development, and an IND application is planned for 2025.

Contact:Miss Jiang
Telephone:028-87014968
Email:xiameijiang@hyperwaypharma.com
Address:8Th Floor, Building B4, Tianfu Life Science Park, No.88, Keyuan South Road, Hi-Tech Zone, Chengdu, Sichuan